Multilingual
Translation workflows
Version Control
Audit history
eTD Data Model
Content based
MS Office
Full integration
Full DMS
Intelligent docs
Impact Analysis
Live tracking
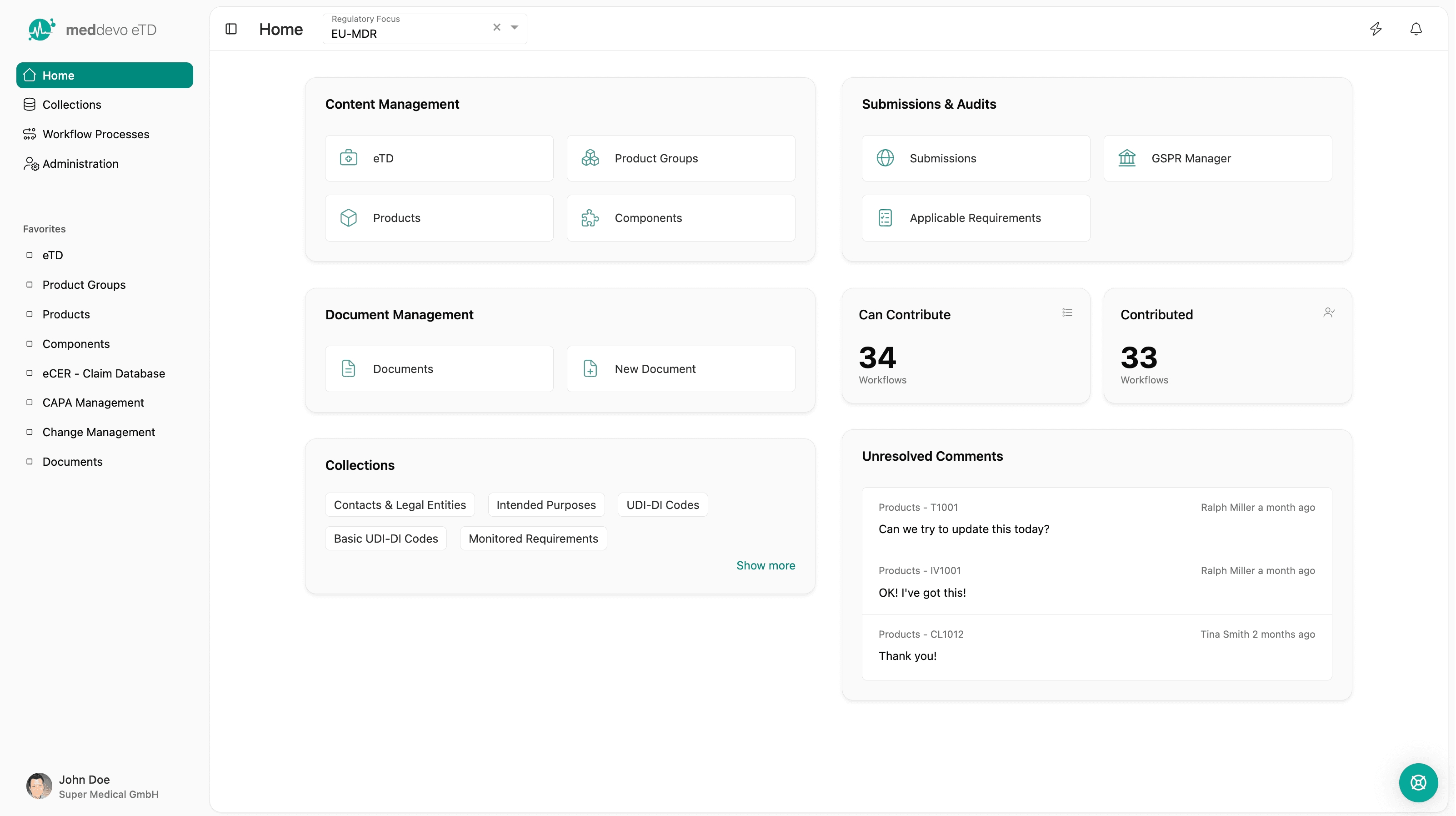
meddevo eTD
Content based technical documentation for medical devices and IVDs
meddevo eTD replaces document driven technical documentation with a structured regulatory data model. Built for medical device and IVD manufacturers scaling portfolios across markets, products and regulations.
Why traditional technical documentation breaks at scale
Technical documentation was never designed for growing regulatory requirements or for scaling portfolios.
Exponential growth of redundancy
Rising manual burden and errors
Longer time to market
Limited transparency
Dependency on individual experts
Document management systems store files.
Quality systems control processes.
Design tools can engineer.
But neither understands regulatory logic and needs.
eTD was built for exactly that.
What eTD changes fundamentally
From documents to structured regulatory data
Instead of editing documents, you manage:
Documents are generated automatically from validated data and stay consistent by design.
This changes regulatory work from reactive document maintenance to controlled data management.
One data model. Full regulatory control.
The meddevo eTD data model has been developed specifically for medical devices and IVDs since 2018 and is preconfigured with regulatory requirements.
Full traceability. Live impact analysis. Always know what changes, where it's used, and which submissions are affected.
Before you submit. Before you audit. Before risk escalates.
What eTD enables
Structured regulatory data instead of static documents
Automated document generation from validated content
Full traceability, impact analysis and document redlining before changes
Enabling secure AI use cases based on data
Platform Modules
eTD Data Model
Multilingual TD content management
medtech DMS
Document management system
eStandards
Standards & MDCG monitoring
eSubmission
Regulatory submissions
UDI Management
Device identification
GSPR Manager
Safety requirements
Risk Management
ISO 14971 compliance
Clinical Evaluation
CER documentation
eTD Data Model
Multilingual TD content management
medtech DMS
Document management system
eStandards
Standards & MDCG monitoring
eSubmission
Regulatory submissions
UDI Management
Device identification
GSPR Manager
Safety requirements
Risk Management
ISO 14971 compliance
Clinical Evaluation
CER documentation
Discover all modules and capabilities of the eTD platform
Explore all eTD featuresDesigned for real regulatory teams
Enterprise ready by default
meddevo eTD scales from single products to global portfolios.
Explore all featuresPlans that scale with your needs
Find the right plan for your organization.
Starter
Best for Start ups
2-4 Users
- Full eTD data model
- Full DMS + Office Integration
- Digital Approval Workflows
- Intelligent Document Generation
- Compliance Inspector
- In-App Support
- and many more...
- Discover all eTD Features →
PRO
Suitable for small companies and SMEs
From 4 Users
- eSubmissions
- Standard Monitoring
- Custom Processes
- REST API Access
- Dedicated Success Manager
- and many more...
- Discover all eTD Features →
Used by companies like
Enterprise
For large companies and its complexities
From 10 Users
- Unlimited Custom Collections
- Multiple Workspaces
- SSO
- and many more...
- Discover all eTD Features →
Used by companies like
A guided path, not a blind rollout
Every eTD implementation starts with a structured evaluation and onboarding process.
Explore real use cases
Migrate legacy documentation with AI support
Guided by our regulatory experts from day one
Build a validated and scalable setup
Ready to turn technical documentation into a strategic asset?
meddevo eTD is more than a tool.
It is the foundation for digital regulatory operations.
Start where you are. Scale when you are ready.